REVIV Launches Methylene Blue IV Therapy Globally
LONDON, UK. 3rd November 2025
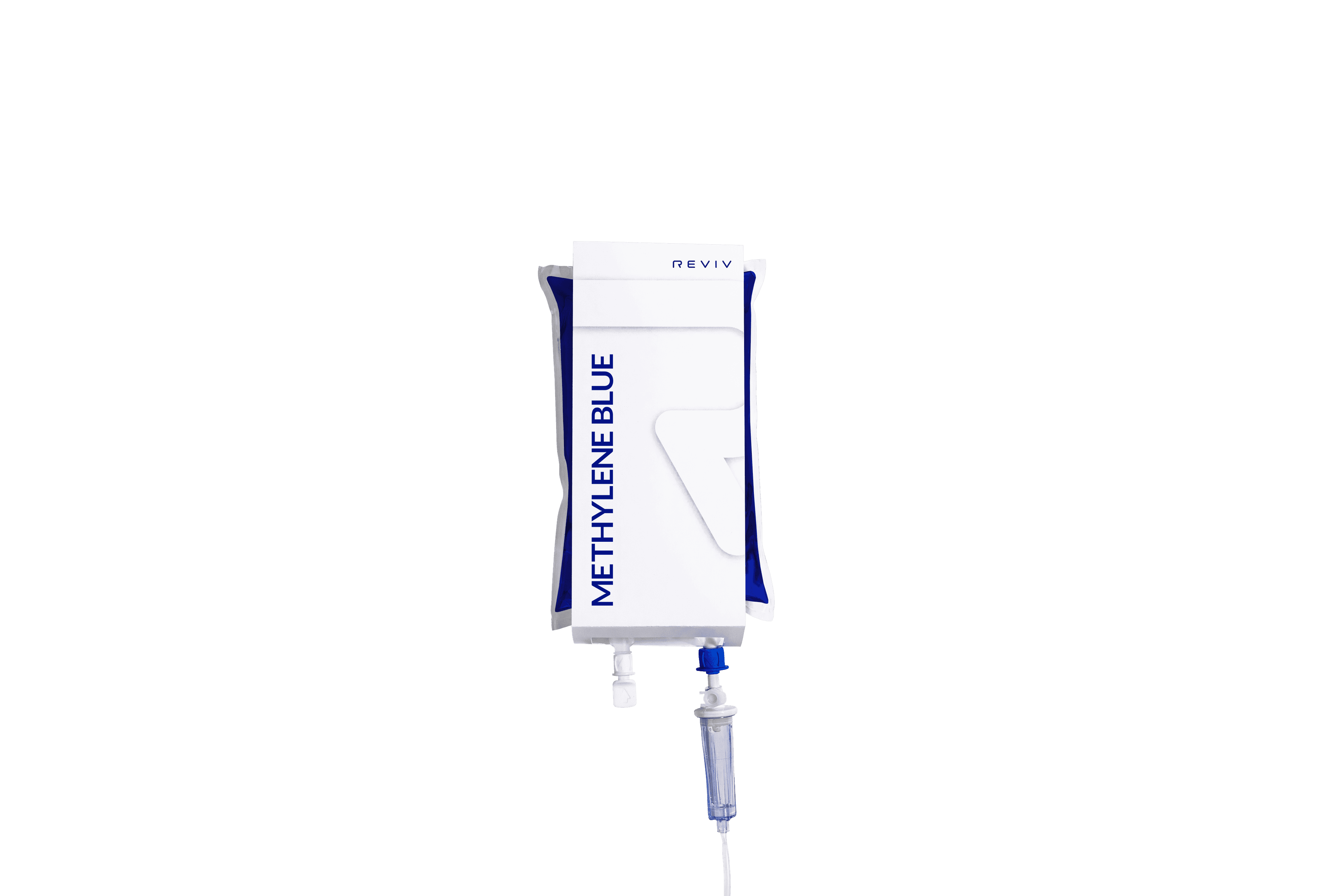
Following its successful launch in the UK and US, REVIV, the global leader in IV therapy and precision health, has now launched its Methylene Blue intravenous (IV) therapy worldwide. The therapy is now available across REVIV’s global clinic network, spanning more than 100 locations in 47 countries.
Methylene Blue IV therapy supports cellular energy production, cognitive function, and recovery from physiological stressors such as travel, intense exercise, and low-oxygen environments. Independently laboratory-tested for safety, the 500ml infusion is administered under trained medical supervision as part of REVIV’s Specialty & Wellness range.
The global rollout of Methylene Blue IV therapy has been extended through REVIV X partners, independent healthcare providers who can offer REVIV therapies using its proprietary protocols, formulations, and technology.
“We’re proud to introduce Methylene Blue as the latest addition to our IV Pro platform,” said Sarah Lomas, CEO of REVIV Global. “This marks another milestone in our commitment to setting global standards for safety, efficacy, and innovation in longevity and regenerative medicine. By equipping our REVIV X partners with the protocols and science-backed frameworks to deliver advanced therapies like Methylene Blue, we move closer to redefining what’s possible in human health optimisation.”
Methylene Blue has been used in medicine since the 19th century, originally to treat blood disorders such as methemoglobinemia. More recently, it is being studied for its ability to help the body produce energy more efficiently at a cellular level, which can support better focus, clearer thinking, and balanced mood. It may also help the body recover from stress caused by factors like travel, intense exercise, or low-oxygen environments.
For media enquiries contact press@revivme.com
Subscribe to our newsletter
Get the latest articles and updates straight to your inbox.
SIMILAR NEWS

Can Glutathione Help With Skin Pigmentation And Psoriasis?
Glutathione Therapy
MAY 21, 2015
Glutathione, commonly referred to as the master anti-oxidant, is a compound produced in the body that is present in various tissues, acting as a neutralizer of toxins and promoting repair.

Hydromax – REVIV’s Pure IV Hydration Therapy
OCTOBER 10, 2016
Intravenous or IV hydration has long been used by people in medical field for restoring fluids, replacing electrolytes, and administering nutrients when there is a barrier to oral intake. Originally reserved only for the severely ill, IV therapy, has over the years become more commonly utilized for preventative purposes and for people looking for fast recovery.
What started out as therapies administered in doctor’s offices are now commonplace in IV bars, med spas and lounges often offering additional therapies such as B12, Glutathione, IV vitamins drips and even IV for hangovers!